Have you read the CDC/NIH BMBL-6 yet? The ABSA International Summary of Changes and the Side-by-Side Comparison will help focus your reading.
The ABSA International Technical and Regulatory Review Committee compiled a Summary of Changes and a Side-by-Side Comparison to the 6th edition of the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL-6) released in November of 2020. The BMBL-6 continues to be, since the first edition in 1984, an advisory document. Consequently, words such as "must" and "should" have been mostly removed. In our review, we found updates to every section of the BMBL, from title changes to added references to include publications since 2009, when the 5th edition of the BMBL was published. We noticed, for example, new references to Zika and Vibrio cholera [...]
ABSA’s Top-5 Biorisk Management Resources
Medical laboratory professionals are recognized at the end of April in the United States. We want to recognize here this group of unsung heroes during the COVID-19 pandemic. Marian Downing, Shoolah Escott, and Michael Marsico will present a poster during the upcoming virtual APHL 2021 Conference showcasing Biorisk Management tips and tools offered by ABSA International that may be of value to the laboratory professional interested in biosafety. Here are the Top-5 tools and resources: Training Tools and resources type "risk assessment" in the search box to retrieve manuscripts, templates, and webinars. In addition, online education is facilitated through webinars [...]
Recent Updates to the SARS-CoV-2 COVID-19 Toolbox by the EIDC
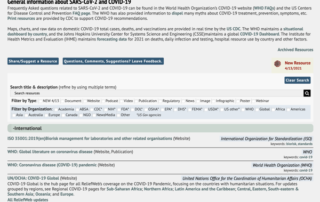
In March 2020, the Emerging Infectious Diseases Committee (EIDC, then a task force) spearheaded the compilation of scientific and non-scientific resources into the SARS-CoV-2 COVID-19 Toolbox. Since then, the Committee has been busy adding resources, updating guidance provided primarily by US CDC and OSHA, and the WHO. But we don’t stop there, members of the EIDC have generated documents including risk assessment considerations for handling COVID-19 related samples in the laboratory setting and for holding community events. Towards the end of 2020, the Committee requested feedback from the ABSA International members and friends on ways to improve the toolbox. After [...]
New COVID-19 Resource: Brochure available on planning events during a pandemic
ABSA International is pleased to provide a brochure to assist organizations with providing a safe and healthy environment during periods when infectious diseases are of concern, such as the current COVID-19 pandemic. The information included serves as a guide for organizations to plan activities involving community members, volunteers, or others where personal interaction is slated to occur. COVID-19 and Your Community: How to plan an event during a pandemic (PDF 2MB)
Mary Ann Liebert, Inc. to Publish Applied Biosafety
For Immediate Release Contact: Kathryn Ryan, Mary Ann Liebert, Inc., publishers, 914-740-2100, kryan@liebertpub.com Mary Ann Liebert, Inc. to Publish Applied Biosafety New Rochelle, NY, July 29, 2020—ABSA International has awarded the contract to publish their journal, Applied Biosafety: Journal of ABSA International, to Mary Ann Liebert, Inc., publishers, effective 2021. The Journal adds a dynamic component to Mary Ann Liebert, Inc.’s renowned portfolio of peer-reviewed journals in biomedical and public health research, including Health Security, Vector-Borne and Zoonotic Diseases, Foodborne Pathogens and Disease, and Viral Immunology. “We are thrilled to partner with ABSA International to publish Applied Biosafety in 2021. [...]
COVID-19 Joint Statement from ABSA International and CABS
May 4, 2020 Dear CABS and ABSA International Members and Partners, As leaders of the Canadian Association for Biological Safety (CABS) and ABSA International, we deeply appreciate the valuable work of our members and partners during this critical response to coronavirus disease (COVID-19). As neighbors, we plan to cultivate a strong relationship and encourage collaboration between the organizations and our members. As we know, viruses don’t respect borders so it is important to work together to ensure good biosafety and biosecurity practices. ABSA International and CABS would like to share the following resources with all of you and plan to [...]
COVID-19 Joint Statement from ABSA International and AALAS
April 16, 2020 Dear AALAS and ABSA International Members and Partners, As leaders of AALAS and ABSA International, we deeply appreciate the valuable work of our members and partners during this critical response to coronavirus disease (COVID-19). Both professions, in most cases working together, are fighting to keep our workplaces and communities safe during the pandemic. ABSA International’s resources in biosafety and biosecurity and AALAS’s educational resources in laboratory animal science are both important to fighting the epidemic. ABSA International and AALAS want to share the following resources with all of you and plan to continue collaboration to assist [...]
ABSA in the News: Embrace experimentation in biosecurity governance
Embrace experimentation in biosecurity governance Sam Weiss Evans, Jacob Beal, Kavita Berger, Diederik A. Bleijs, Alessia Cagnetti, Francesca Ceroni, Gerald L. Epstein, Natàlia Garcia-Reyero, David R. Gillum, Graeme Harkess, Nathan J. Hillson, Petra A. M. Hogervorst, Jacob L. Jordan, Geneviève Lacroix, Rebecca Moritz, Seán S. ÓhÉigeartaigh, Megan J. Palmer and Mark W. J. van Passel Science 368 (6487), 138-140. DOI: 10.1126/science.aba2932 Science Vol 368, Issue 6487 10 April 2020 Summary As biological research and its applications rapidly evolve, new attempts at the governance of biology are emerging, challenging traditional assumptions about how science works and who is responsible for [...]